Top videos
Hank does his best to convince us that chemistry is not torture, but is instead the amazing and beautiful science of stuff. Chemistry can tell us how three tiny particles - the proton, neutron, and electron - come together in trillions of combinations to form ... everything. In this inaugural episode of Crash Course Chemistry, we start out with one of the biggest ideas in chemistry ever - stuff is made from atoms. More specifically, we learn about the properties of the nucleus and why they are important to defining what an atom actually is.
This video is available in Spanish on our Crash Course en Español channel! Watch it here: https://youtu.be/G7wUMpsB5k8
Pssst... we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: https://apple.co/3d4eyZo
Download it here for Android Devices: https://bit.ly/2SrDulJ
Table of Contents
Intro 00:00
Einstein & Atoms 02:05
Composition of Atoms 03:18
Atomic Number 04:20
Isotopes 08:04
Relative Atomic Mass 07:26
Mass Number 07:44
Watch the SciShow episodes on the Strong Nuclear Force here:
http://www.youtube.com/watch?v=Yv3EMq2Dgq8
http://www.youtube.com/watch?v=BNDOSMqGLlg
Crash Course is on Patreon! You can support us directly by signing up at http://www.patreon.com/crashcourse
Want to find Crash Course elsewhere on the internet?
Facebook - http://www.facebook.com/YouTubeCrashCourse
Twitter - http://www.twitter.com/TheCrashCourse
Instagram - https://www.instagram.com/thecrashcourse/
CC Kids: http://www.youtube.com/crashcoursekids
This video tutorial provides a basic introduction into chemistry. You can access the full video at the link shown below:
Full Video on Patreon: https://www.patreon.com/MathScienceTutor
Direct Link to The Full Video:
https://bit.ly/3hIRJCx
___________________________
Full 1 Hour 42 Minute Video:
https://www.youtube.com/watch?v=oK3XF1gdPGM
Join The Membership Program:
https://bit.ly/46xaQTR
Here is a list of topics:
1. Intro to the Periodic Table of the Elements
2. Alkali Metals, Alkaline Earth Metals, Transition Metals, Chalcogens, Halogens, and Noble Gases
3. Ion Charges of Representative Elements
4. Atoms, Molecules, Pure Elements, and Compounds
5. Ionic Compounds and Molecular Compounds
6. Metals, Nonmetals, and Metalloids
7. Atoms vs Ions
8. Cations and Anions
9. Nomenclature of Molecular Compounds
10. How To Name Ionic Compounds
11. Naming Ionic Compounds With Transition Metals & Roman Numerals
12. Polyatomic Ions
13. Writing Chemical Formulas of Ionic Compounds
14. Average Atomic Mass
15. Isotopes
16. How To Determine The Number of Protons, Neutrons, and Electrons In Atoms and Ions
In this video we read about :----
introduction
sources of water
impurities in water
sources of impurities in water
and specification of water for different uses
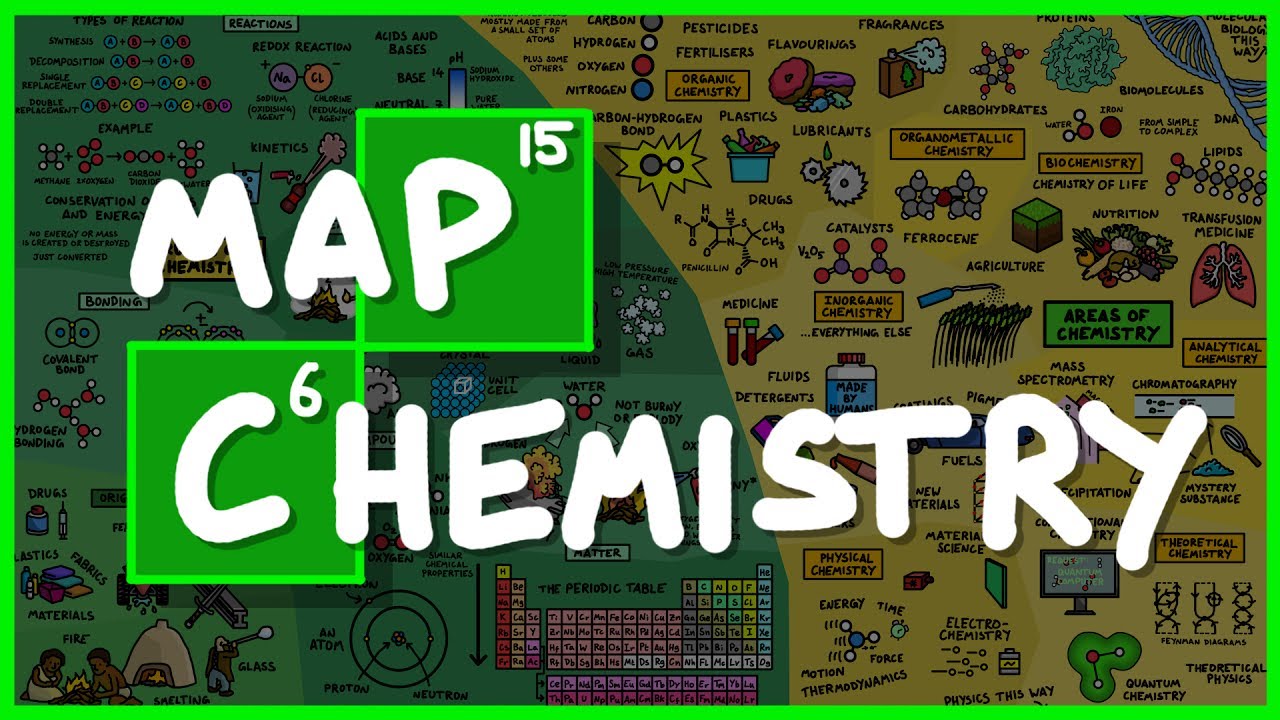
The entire field of chemistry summarised in 12mins from simple atoms to the molecules that keep you alive.
#chemistry #DomainOfScience
If you would like to buy a poster of this map, they are available here: https://www.redbubble.com/peop....le/dominicwalliman/w
I have also made a version available for educational use which you can find here: https://flic.kr/p/UBS4mf
and a widescreen version: https://flic.kr/p/UNA1LW
Errata and notes:
1. I got the Oxidising Agent and the Reducing Agent the wrong way around! Sodium is the Reducing agent and Chlorine is the Oxidising agent. My confusion was that when a sodium atom looses an electron it becomes oxidised, so in my simple brain, I called it the oxidising agent. That is wrong because the agent that oxidises the sodium is the chlorine atom and so the labels are the wrong way around. Doh!
2. I drew the hydrogen H2 molecule with a double bond but it should be a single bond because they are bonded with a single covalent bond.
3. Where I have drawn carbon dioxide, the carbon should have a double bond to each of the oxygens.
4. Apparently Feynman diagrams are not that useful for theoretical chemistry, so perhaps that wasn't the best choice for the illustration. The feedback in the comments from a real theoretical chemist is "All we deal with is shuffling around electrons, but many many many electrons, so a Feynman diagram would need to be huge but at the same time would be very very repetitive."
5. In analytical chemistry, I should have called it distillation rather than precipitation.
6. My definition of organic chemistry being about ‘life’ is not very good. I should have said that organic chemistry looks at compounds that contain carbon. But there are some compounds in inorganic chemistry that also contain carbon, like carbon dioxide so I guess I'd also have to state that inorganic chemistry is almost everything else.
7. I said that fuels are inorganic chemistry which is misleading when I drew a car next to it. My understanding is that there are inorganic fuels that don't contain carbon, but obviously all the fuels we are familiar with are organic. I thought a picture of a car would tie a few things together elegantly, but it ended up giving the wrong impression. That’s okay, I’m still learning! :D
8. In inorganic chemistry, I should have stated that all natural minerals fall under inorganic chemistry so as not to be misleading, otherwise you might go way thinking that only man-made substances fall under inorganic chemistry which is not true. I said that 'a lot of the inorganic compounds that are studied are man-made' meaning that the cutting edge of research is mostly man-made substances.
9. Apparently water is not the most inflammable substance. I thought it was so that is interesting.
10. In the bonding section, hydrogen bonding and van der waals forces are technically inter molecular forces.
Here are some of the references I used for this video if you’d like to dig a little deeper
https://en.wikipedia.org/wiki/Chemistry
https://en.wikipedia.org/wiki/Outline_of_chemistry
https://www.uwlax.edu/chemistr....y-and-biochemistry/s
https://www.khanacademy.org/science/chemistry
https://www.cancerquest.org/ca....ncer-biology/biologi
Early smelting:
http://ispatguru.com/evolution....-of-blast-furnace-ir
Categorisation of reactions
http://www2.ucdsb.on.ca/tiss/s....tretton/chem1/stoich
Thanks so much to my supporters on Patreon. If you enjoy my videos and would like to help me make more this is the best way and I appreciate it very much. https://www.patreon.com/domainofscience
Frontiers of Space: http://nobrow.net/shop/profess....or-astro-cats-fronti
Atomic Adventure: http://nobrow.net/shop/profess....or-astro-cats-atomic
Intergalactic Activity Book: http://nobrow.net/shop/profess....or-astro-cats-interg
Solar System App: http://www.minilabstudios.com/....apps/professor-astro
Find me on twitter, instagram, and my website:
http://dominicwalliman.com
https://twitter.com/DominicWalliman
https://www.instagram.com/dominicwalliman
https://www.facebook.com/dominicwalliman
Chad gives a comprehensive lesson on how to rank acids and bases. This is presented in the context of the ARIO mnemonic which stands for Atom, Resonance, Induction, and Orbitals which helps students remember the most important factors affecting acidity and basicity in the most common order of importance. The Atom Rule for ranking acids and bases shows how the size and electronegativity of the basic atom affect its strength as a base. Of second importance is the effect of resonance on acidity and basicity as resonance can stabilize a base resulting in a weaker base. Typically of third importance is the effect of induction on acidity and basicity. Electron-withdrawing groups such as electronegative atoms near the atom acting as a base will help stabilize it resulting in a weaker base. Finally, the Orbital Rule describes the role that the hybridization of the atom acting as a base affects its basicity. Generally and all else being equal the order of stability with regard to hybridization is sp / sp2 / sp3 and the order of base strength is the opposite: sp3 / sp2 / sp. Chad provides numerous examples of how to rank acids and bases to demonstrate how each rule is applied, and concludes the lesson with several additional examples of ranking acids.
If you want all my study guides, quizzes, and practice exams, check out my premium course at https://www.chadsprep.com/orga....nic-chemistry-course
00:00 Lesson Introduction
00:33 Ranking Acids and Bases (ARIO mnemonic)
03:31 The Effect of Charge on Acidity and Basicity of Organic Compounds
06:10 The Atom Rule for Ranking Acids and Bases of Organic Compounds
12:27 How Resonance Affects Acidity and Basicity of Organic Compounds
16:20 How Induction Affects Acidity and Basicity of Organic Compounds
25:12 How Hybridization Affects Acidity and Basicity of Organic Compounds
29:09 Examples of Ranking Acids
https://www.chadsprep.com/
Are you confused by IUPAC nomenclature? Let Professor Dave help! First I'll explain what line notation represents, and then I'll introduce the basic rules for naming alkanes.
Watch the whole Organic Chemistry playlist: http://bit.ly/ProfDaveOrgChem
General Chemistry Tutorials: http://bit.ly/ProfDaveGenChem
Biochemistry Tutorials: http://bit.ly/ProfDaveBiochem
Biology Tutorials: http://bit.ly/ProfDaveBio
Classical Physics Tutorials: http://bit.ly/ProfDavePhysics1
Modern Physics Tutorials: http://bit.ly/ProfDavePhysics2
Mathematics Tutorials: http://bit.ly/ProfDaveMath
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► http://patreon.com/ProfessorDaveExplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: https://amzn.to/2HtNpVH
Bookshop: https://bit.ly/39cKADM
Barnes and Noble: https://bit.ly/3pUjmrn
Book Depository: http://bit.ly/3aOVDlT
The course I'm teaching is a distilled version of the material covered in the following textbooks, which I highly reccommend:
Inorganic Chemistry: https://amzn.to/4cW8v82
Chemistry, 5th addtion: https://amzn.to/3W0EQDS
This video is a simple introduction to inorganic chemistry and its applications. It's the first part of a series that teaches the basics, starting with core concepts. Please note that the information in these videos may not always be accurate or up-to-date due to new discoveries. So, only use them as additional resources to improve your understanding.
- inorganic chemistry
- chemistry
- what is inorganic chemistry
- chemistry course
- learn chemistry course
-
UCI Chemistry: Inorganic Chemistry (Fall 2014)
Lec 02. Inorganic Chemistry -- Symmetry and Point Groups
View the complete course: http://ocw.uci.edu/courses/che....m_107_inorganic_chem
Instructor: Matthew D. Law
License: Creative Commons CC-BY-SA
Terms of Use: http://ocw.uci.edu/info
More courses at http://ocw.uci.edu
Description: This course is an introduction to modern inorganic chemistry. Topics include principles of structure, bonding, and chemical reactivity with application to compounds of the main group and transition elements, including organometallic chemistry.
Inorganic Chemistry (Chem 107) is part of OpenChem: http://ocw.uci.edu/collections/open_chemistry.html
This video is part of a 29-lecture undergraduate-level course titled "Inorganic Chemistry" taught at UC Irvine by Professor Matthew D. Law.
Recorded on October 6, 2014.
Index of Topics:
00:59-Symmetry in Molecules: Staggered Ethane
19:20-Summary
21:44-Low-Symmetry Point Groups
24:38-High-Symmetry Point Groups
29:42-Linear Point Groups
31:53-D Point Groups
36:09-C Point Groups
38:35-S Point Groups
41:01-Identifying Point Groups
43:45-Example: Phosphorous Pentafluoride
46:33-Example: Diborane
48:17-Example: 18-crown-6 Ether
Required attribution: Law, Matthew D. Inorganic Chemistry 107 (UCI OpenCourseWare: University of California, Irvine), http://ocw.uci.edu/courses/che....m_107_inorganic_chem. [Access date]. License: Creative Commons Attribution-ShareAlike 3.0 United States License. (http://creativecommons.org/lic....enses/by-sa/3.0/deed
UCI Chemistry: Inorganic Chemistry (Fall 2014)
Lec 01. Inorganic Chemistry -- Course Introduction & Symmetry of Nature
View the complete course: http://ocw.uci.edu/courses/che....m_107_inorganic_chem
Instructor: Matthew D. Law
License: Creative Commons CC-BY-SA
Terms of Use: http://ocw.uci.edu/info
More courses at http://ocw.uci.edu
Description: This course is an introduction to modern inorganic chemistry. Topics include principles of structure, bonding, and chemical reactivity with application to compounds of the main group and transition elements, including organometallic chemistry.
Inorganic Chemistry (Chem 107) is part of OpenChem: http://ocw.uci.edu/collections/open_chemistry.html
This video is part of a 29-lecture undergraduate-level course titled "Inorganic Chemistry" taught at UC Irvine by Professor Matthew D. Law.
Recorded on October 3, 2014.
Index of Topics:
00:08-Symmetry in Nature
01:30-Symmetry Sitting Next to You
01:59-Symmetry Elements and Operations
06:48-The Identity
07:48-Proper Rotations
18:24-Reflections
18:31-Proper Rotations
19:32-Reflections
22:40-Inversion
28:10-Improper Rotations
Required attribution: Law, Matthew D. Inorganic Chemistry 107 (UCI OpenCourseWare: University of California, Irvine), http://ocw.uci.edu/courses/che....m_107_inorganic_chem. [Access date]. License: Creative Commons Attribution-ShareAlike 3.0 United States License. (http://creativecommons.org/lic....enses/by-sa/3.0/deed
Best Technique to study Inorganic Chemistry को सबसे strong करने के लिए DNC Technique
𝐀𝐛𝐨𝐮𝐭 𝐏𝐫𝐚𝐭𝐞𝐞𝐤 𝐒𝐢𝐫:
👉 IIT Bombay (B. Tech)
👉 Youth Motivator, Mentored Lakhs of Students
👉 5+ Years of Teaching Experience
👉 Expert of Online Teaching & Content Visualization
𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐞𝐒𝐚𝐫𝐚𝐥 𝐀𝐩𝐩 𝐟𝐨𝐫 𝐉𝐄𝐄, 𝐍𝐄𝐄𝐓, 𝐂𝐥𝐚𝐬𝐬 𝟗 & 𝟏𝟎 𝐂𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐂𝐨𝐮𝐫𝐬𝐞𝐬.
📱 𝗲𝗦𝗮𝗿𝗮𝗹 𝗔𝗽𝗽 𝗗𝗼𝘄𝗻𝗹𝗼𝗮𝗱 𝗟𝗶𝗻𝗸: http://bit.ly/eSaralApp
📞𝐅𝐨𝐫 𝐦𝐨𝐫𝐞 𝐝𝐞𝐭𝐚𝐢𝐥𝐬, 𝐜𝐨𝐧𝐭𝐚𝐜𝐭 𝐡𝐞𝐫𝐞: +91-6376440597, +91-9024464479
#eSaral_Learning #eSaral_JEE #eSaral_NEET
🔰S2A 2024 Chemistry Main Telegram Channel:
https://t.me/S2Achemistrymain
🔰S2A 2024 Physics Main Telegram Channel:
https://t.me/S2Aphysicsmain
🔰S2A Academy Whatsapp Channel:
https://whatsapp.com/channel/0....029VaHBig2BKfhwlcVg2