Education
Sub Category
In this video we cover Basic Organic Chemistry Concepts in full.
Chad gives a comprehensive lesson on how to rank acids and bases. This is presented in the context of the ARIO mnemonic which stands for Atom, Resonance, Induction, and Orbitals which helps students remember the most important factors affecting acidity and basicity in the most common order of importance. The Atom Rule for ranking acids and bases shows how the size and electronegativity of the basic atom affect its strength as a base. Of second importance is the effect of resonance on acidity and basicity as resonance can stabilize a base resulting in a weaker base. Typically of third importance is the effect of induction on acidity and basicity. Electron-withdrawing groups such as electronegative atoms near the atom acting as a base will help stabilize it resulting in a weaker base. Finally, the Orbital Rule describes the role that the hybridization of the atom acting as a base affects its basicity. Generally and all else being equal the order of stability with regard to hybridization is sp / sp2 / sp3 and the order of base strength is the opposite: sp3 / sp2 / sp. Chad provides numerous examples of how to rank acids and bases to demonstrate how each rule is applied, and concludes the lesson with several additional examples of ranking acids.
If you want all my study guides, quizzes, and practice exams, check out my premium course at https://www.chadsprep.com/orga....nic-chemistry-course
00:00 Lesson Introduction
00:33 Ranking Acids and Bases (ARIO mnemonic)
03:31 The Effect of Charge on Acidity and Basicity of Organic Compounds
06:10 The Atom Rule for Ranking Acids and Bases of Organic Compounds
12:27 How Resonance Affects Acidity and Basicity of Organic Compounds
16:20 How Induction Affects Acidity and Basicity of Organic Compounds
25:12 How Hybridization Affects Acidity and Basicity of Organic Compounds
29:09 Examples of Ranking Acids
https://www.chadsprep.com/
This video explains basics of orgranic chemistrty. Main topics of organic chemistry, such as functional groups, isomerism, hybridization, bonding, and reaction of organic compounds, are explained in a very easy and effective way. This video is helpful for students and learners of chemistry every age.
organic chemistry explained
Organic chemistry for beginners
General chemistry explained
organic chemistry
General Chemistry Explained in 19 minutes
Wacky Science
GCSE Chemistry
Timestamps:
00:00 Introduction
00:18 Vital Force Theory
00:38 Modern Definition
00:48 Catenation
01:05 Classification of Organic Compounds
01:49 Closed Chain Compounds
02:30 Aromatic Compounds
02:38 Functional Groups
03:26 Isomerism
04:19 Stereoisomerism
04:54 Hybridization
05:17 sp Hybridization
05:46 sp² Hybridization
06:15 sp³ Hybridization
06:42 Bonding in Organic Compounds
07:10 Organic Reactions
Crash course
What is organic chemistry
Organic chemistry tutorial
Basic orgranic chemistry
organic chemistry tutor
organic chemistry introduction
organic chemistry reactions
Organic chemistry
iupac
how to prepare for organic chemistry
isomers,
organic chemistry crash course
Functional group
Isomerism
Hybridization
Hydrocabons
Alkanes
Alkenes
Alkynes
Cis trans isomerism
#organicchemistry
#chemistry
#organiccompound
Naming alkanes are broken down into just 3 steps! In this video I will go over the five common cases you will see on quizzes and exams, so you don't lose any points on your next organic chemistry exam. 🤓
📘 FREE ORGANIC CHEMISTRY SURVIVAL GUIDE
https://melissa.help/freeochemguide
💯 HERE'S HOW TO PASS ORGANIC CHEMISTRY 🎉
https://chemmunity.info/youtube
👉 MORE CHEMISTRY RESOURCES I CREATED 👈
https://melissamaribel.com/
🎓 CHECKOUT MY COMPLETE CHEMISTRY GUIDES:
📕 Thermochemistry Guide
https://melissa.help/thermonotes
📗 Acids and Bases Guide
https://melissa.help/acidbase1notes
📘 Naming Compounds and Acids Guide
https://melissa.help/namingnotes
📙 Dimensional Analysis, Significant Figures, and Density Guide
https://melissa.help/sigfignotes
📕 Gas Laws Guide
https://melissa.help/gaslawsnotes
📗 Stoichiometry Guide
https://melissa.help/stoichnotes
📘 Redox Reactions Guide
https://melissa.help/redoxnotes
📙 Molarity Guide
https://melissa.help/molaritynotes
📕 Limiting Reactants Guide
https://melissa.help/limreactnotes
📗 Lewis Structures Guide
https://melissa.help/lewisnotes
📘 Kinetics Guide
https://melissa.help/kineticsnotes
📙 Titrations Guide
https://melissa.help/titrations
📕 Matter, Atomic Structure, Empirical and Molecular Formulas Guide
https://melissa.help/matterguide
🙌 This was my go-to homework help when I was in school. Chegg Study is one of my favorites.
https://melissa.help/cheggstudy
📚 I made the mistake of buying all of my textbooks, I wish I had the option of renting them. Thankfully you do, with Chegg Textbook Rentals.
https://melissa.help/cheggbooks
💁♀️ HI I'M MELISSA MARIBEL
I help students pass Chemistry and Organic Chemistry. I used to struggle with this subject, so when I finally graduated with a bachelor's degree in Chemistry, I became a tutor so that you wouldn't have to struggle like I did. I know that with the right help, YOU CAN LEARN ANYTHING!
DISCLAIMER: Some links in the description are affiliate links, which means that if you buy from those links, I’ll receive a small commission. This helps support the channel and allows me to continue making videos like this. Thanks for the support!
Chad gives a comprehensive lesson on Resonance, showing organic chemistry students exactly how to draw resonance structures easily. He starts by reviewing what students would have learned in General Chemistry including the idea that resonance implies delocalized electrons which typically results in greater stability. He then covers numerous resonance structure examples relevant to organic chemistry including resonance with carbocations, resonance with lone pairs of electrons, resonance with radicals, and how to draw resonance structures with arrows. He also shows how to predict what the structure of the resonance hybrid should look like and how to determine which resonance structures are major or minor contributors. Finally, Chad shows the relationship between resonance and hybridization, and how to properly determine the hybridization of an atom when resonance is a factor.
If you want all my study guides, quizzes, and practice exams, check out my premium course at https://www.chadsprep.com/orga....nic-chemistry-course
00:00 Lesson Introduction
00:23 Introduction to Resonance
05:02 Drawing Resonance Structures (Arrow-Pushing)
11:29 Drawing Resonance Structures for Stabilizing Carbocations
16:22 Drawing Resonance Structures for Stabilizing Lone Pairs
20:30 Drawing Resonance Structures for Stabilizing Radicals
22:19 Determining Major and Minor Resonance Contributors
27:08 Additional Examples with Drawing Resonance Structures
36:18 Resonance and Hybridization
https://www.chadsprep.com/
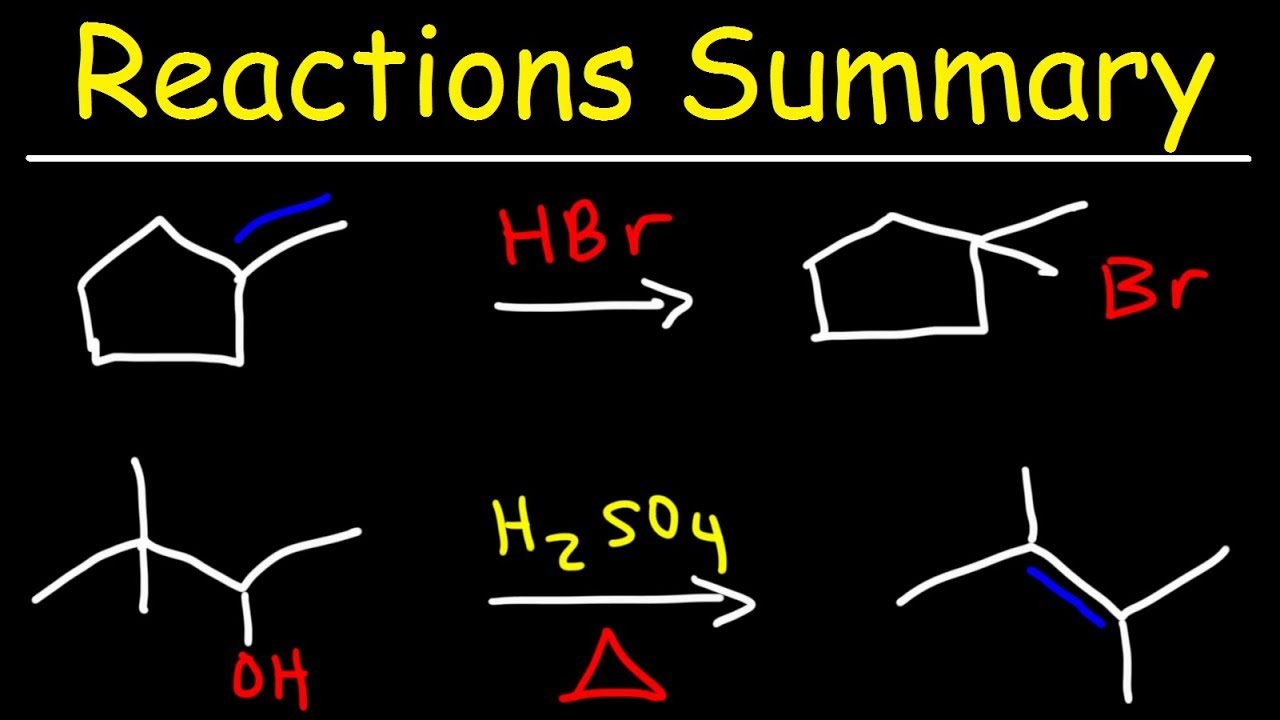
This organic chemistry video tutorial provides a basic introduction into common reactions taught in the first semester of a typical college course of organic chemistry.
Alcohol Reactions - HBr, PBr3, & SOCl2: https://www.youtube.com/watch?v=4PDs3ygNsv4
Free Radical Reactions:
https://www.youtube.com/watch?v=w9RAULFkqKQ
Organic Chemistry 1 Final Exam Review:
https://www.youtube.com/watch?v=ej2pSWw6U3w
IR Spectroscopy:
https://www.youtube.com/watch?v=WTmj_9VT5oE
Mass Spectrometry:
https://www.youtube.com/watch?v=VUIPYnWLSDE
______________________________
Proton NMR Spectroscopy:
https://www.youtube.com/watch?v=vejKDb1dBn8
Carbon-13 NMR Spectroscopy:
https://www.youtube.com/watch?v=QUVTATcXE9Y
Ethers and Epoxides:
https://www.youtube.com/watch?v=BxRLJhHjqMc
Diels Alder Reaction:
https://www.youtube.com/watch?v=sBmoP6KwK-8
Organic Chemistry 2 Final Exam Review:
https://www.youtube.com/watch?v=T5NaUdVfAOc
This lesson introduces you to organic chemistry part 1.
For online tuition, contact me on my whatsapp line 0977924175.
For donation to the channel of any amount, use my mobile line 0969107073. Thank you for your support.
IMPORTANT MESSAGE:
Check for my book on Amazon, it is entitled " Chemistry for Secondary School" an introduction to chemistry. Its available on Amazon kindle. Check for it by click on the link below to be able to read the first chapters for free. Please let me know your feedback, thank you:
https://amzn.to/3jiP13C
This organic chemistry 1 final exam review is for students taking a standardize multiple choice exam at the end of their semester. This review contains plenty of examples and practice problems.
Full 6 Hour Video on Patreon: https://www.patreon.com/MathScienceTutor
Direct Link to The Full Video:
https://bit.ly/2WCJ8GP
Download Exam - 100 Questions:
https://bit.ly/3ojGrYc
Organic Chem. PDF Worksheets:
https://www.video-tutor.net/orgo-chem.html
______________________________
Full Video on YouTube - Part 2:
https://www.youtube.com/watch?v=g5ccmfLHgew
Full Video on YouTube - Part 3:
https://www.youtube.com/watch?v=O8giR03wjCQ
Join The Membership Program:
https://bit.ly/46xaQTR
The Functional Group Concept Explained | Organic Chemistry | FuseSchool
This is an introduction to the Functional Group concept - providing an overview of Organic Chemistry, the composition of Alkenes.
SUPPORT US ON PATREON
https://www.patreon.com/fuseschool
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
VISIT us at [a]www.fuseschool.org%2C[/a] where all of our videos are carefully organised into topics and specific orders, and to see what else we have on offer. Comment, like and share with other learners. You can both ask and answer questions, and teachers will get back to you.
These videos can be used in a flipped classroom model or as a revision aid.
Find all of our Chemistry videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Find all of our Biology videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Find all of our Physics videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Find all of our Maths videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Instagram: https://www.instagram.com/fuseschool/
Facebook: https://www.facebook.com/fuseschool/
Twitter: https://twitter.com/fuseSchool
Access a deeper Learning Experience in the FuseSchool platform and app: www.fuseschool.org
Follow us: http://www.youtube.com/fuseschool
Befriend us: http://www.facebook.com/fuseschool
This is an Open Educational Resource. If you would like to use the video, please contact us: info@fuseschool.org
Introduction to Organic Chemistry Gr 12
Do you need more videos? I have a complete online course with way more content.Click here: https://purchase.kevinmathandscience.com/299course
🟢Follow me on Instagram: https://www.instagram.com/kevinmathscience
Struggling with naming organic compounds? Well, look no further... After you've watched this lesson, sho should be in a posttion to draw structural and condensed structural formulae.
When we venture to new places, we need navigational tools to guide us. In organic chemistry, those are reaction mechanisms! In this episode of Crash Course Organic Chemistry, we’ll learn all about how to write reaction mechanisms. Having this super useful skill means we don’t have to worry about memorizing every reaction that has ever existed.
Series Sources:
Brown, W. H., Iverson, B. L., Ansyln, E. V., Foote, C., Organic Chemistry; 8th ed.; Cengage Learning, Boston, 2018.
Bruice, P. Y., Organic Chemistry, 7th ed.; Pearson Education, Inc., United States, 2014.
Clayden, J., Greeves, N., Warren., S., Organic Chemistry, 2nd ed.; Oxford University Press, New York, 2012.
Jones Jr., M.; Fleming, S. A., Organic Chemistry, 5th ed.; W. W. Norton & Company, New York, 2014.
Klein., D., Organic Chemistry; 1st ed.; John Wiley & Sons, United States, 2012.
Louden M., Organic Chemistry; 5th ed.; Roberts and Company Publishers, Colorado, 2009.
McMurry, J., Organic Chemistry, 9th ed.; Cengage Learning, Boston, 2016.
Smith, J. G., Organic chemistry; 6th ed.; McGraw-Hill Education, New York, 2020.
Wade., L. G., Organic Chemistry; 8th ed.; Pearson Education, Inc., United States, 2013.
***
Watch our videos and review your learning with the Crash Course App!
Download here for Apple Devices: https://apple.co/3d4eyZo
Download here for Android Devices: https://bit.ly/2SrDulJ
Crash Course is on Patreon! You can support us directly by signing up at http://www.patreon.com/crashcourse
Thanks to the following patrons for their generous monthly contributions that help keep Crash Course free for everyone forever:
Catherine Conroy, Patty Laqua, Leonora Rossé Muñoz, Stephen Saar, John Lee, Phil Simmons, Alexander Thomson, Mark & Susan Billian, Junrong Eric Zhu, Alan Bridgeman, Jennifer Smith, Matt Curls, Tim Kwist, Ron Lin, Jonathan Zbikowski. Jennifer Killen, Sarah & Nathan Catchings, Brandon Westmoreland, team dorsey, Trevin Beattie, Eric Prestemon, Sam Ferguson, Yasenia Cruz, Eric Koslow, Indika Siriwardena, Khaled El Shalakany, Shawn Arnold, Tom Trval, Siobhán, Ken Penttinen, Nathan Taylor, William McGraw, Justin Zingsheim, Andrei Krishkevich, Jirat, Brian Thomas Gossett, SR Foxley, Ian Dundore, Jason A Saslow, Jessica Wode, Mark, Caleb Weeks, Sam Buck
--
Want to find Crash Course elsewhere on the internet?
Facebook - http://www.facebook.com/YouTubeCrashCourse
Twitter - http://www.twitter.com/TheCrashCourse
Tumblr - http://thecrashcourse.tumblr.com
Support Crash Course on Patreon: http://patreon.com/crashcourse
CC Kids: http://www.youtube.com/crashcoursekids
This organic chemistry video tutorial provides a basic introduction into naming organic compounds. It explains how to write the IUPAC nomenclature of alkanes. It covers the basic iupac rules such as numbering and writing the substituents in alphabetical order. It explains the importance of identifying the parent chain or the longest carbon chain as well as identifying the parent chain with highest number of substituents.
Access The Full 42 Minute Video:
https://www.patreon.com/MathScienceTutor
Direct Link to The Full Video:
https://bit.ly/3GURyOl
Organic Chemistry PDF Worksheets:
https://www.video-tutor.net/orgo-chem.html
_______________________________________
Join The YouTube Membership Program:
https://bit.ly/46xaQTR
Full 42 Minute Video on YouTube:
https://www.youtube.com/watch?v=tqn1drpHh4c
Contact me in Telegram Using the Link;
https://t.me/kcsebio
.
For more Revision and Past Papers, you may join my Telegram Channels for Biology and Chemistry using the following links;
.
Telegram Biology;
t.me/KCSEBiology
Telegram Chemistry;
t.me/KCSEChemistry
.
Feel free to ask any question in the comment section, or through the channels email;
kcsebiologymasters@gmail.com
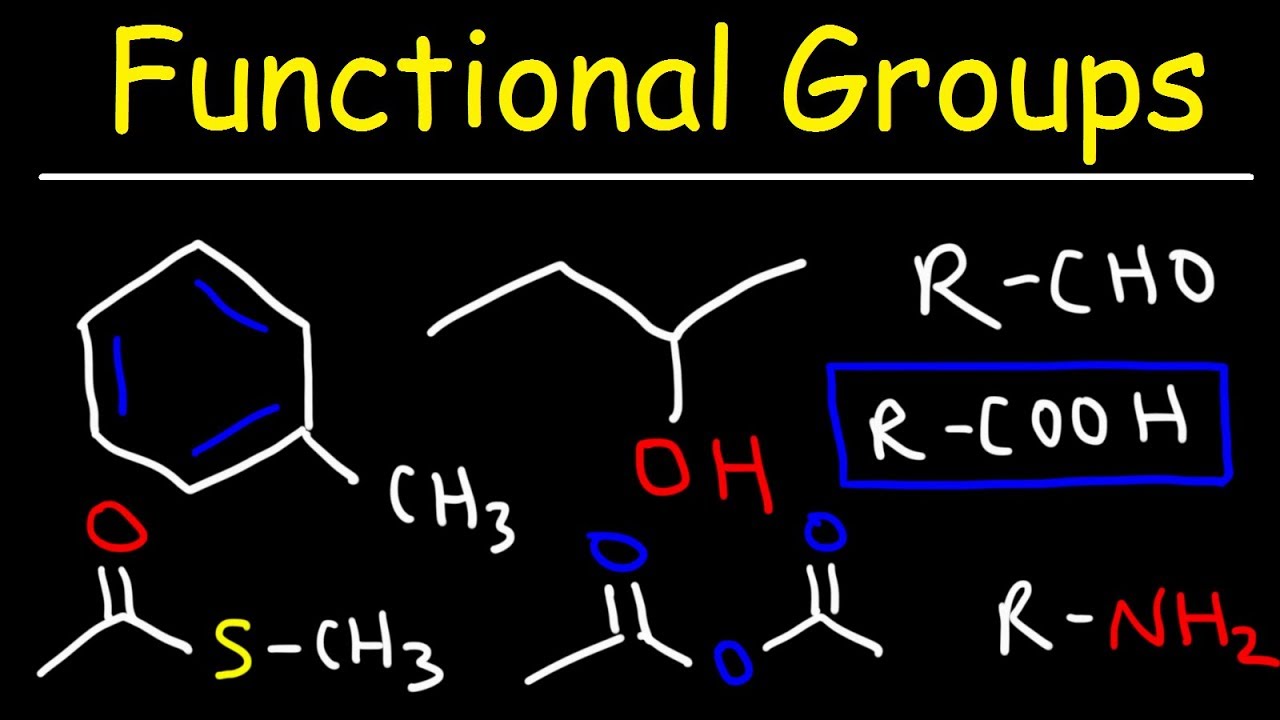
This organic chemistry video tutorial provides a basic introduction into functional groups. It covers alkanes, alkenes, alkynes, aromatic rings, alcohols, ethers, esters, carboxylic acids, ketones, aldehydes, acid chlorides, acid anhydrides, amines, amides, nitriles, thiols, thioethers, thioesters, enols, imines, enamines, carbocations, carbanions, radicals, and carbenes just to name a few.
Organic Chemistry - Basic Introduction:
https://www.youtube.com/watch?v=B_ketdzJtY8
Which Bond Is More Polar?
https://www.youtube.com/watch?v=o0-a5HzSzdE
How To Draw Lewis Structures:
https://www.youtube.com/watch?v=6unef5Hz6SU
Condensed Structures to Skeletal Structures:
https://www.youtube.com/watch?v=HRkvjKHFNDA
Functional Groups Review:
https://www.youtube.com/watch?v=m9jM8lWxrAE
Primary, Secondary, & Tertiary Functional Groups:
https://www.youtube.com/watch?v=r_Zhb0nQEvE
_________________________________
How To Calculate Formal Charge:
https://www.youtube.com/watch?v=C2l-76VP8s0
Finding Lone Pairs Using Formal Charge:
https://www.youtube.com/watch?v=jlCPY6iXQ1c
Dipole Moment & Electronegativity:
https://www.youtube.com/watch?v=bFAU1GMJmnI
Predicting Bond Angles:
https://www.youtube.com/watch?v=DPS7zdg8HzY
Valence Bond Theory:
https://www.youtube.com/watch?v=Vqx9a2aU99c
Hybridization of Atomic Orbitals:
https://www.youtube.com/watch?v=pdJeQUd2g_4
_______________________________
Bond Strength and Bond Length:
https://www.youtube.com/watch?v=SSRY95IAwF8
Orbital Overlap and Bond Length:
https://www.youtube.com/watch?v=BatJrR5sblA
Organic Chemistry PDF Worksheets:
https://www.video-tutor.net/orgo-chem.html
Organic Chemistry Exam 1 Playlist:
https://bit.ly/3kJnNXU
Full-Length Videos and Worksheets:
https://www.patreon.com/MathSc....ienceTutor/collectio
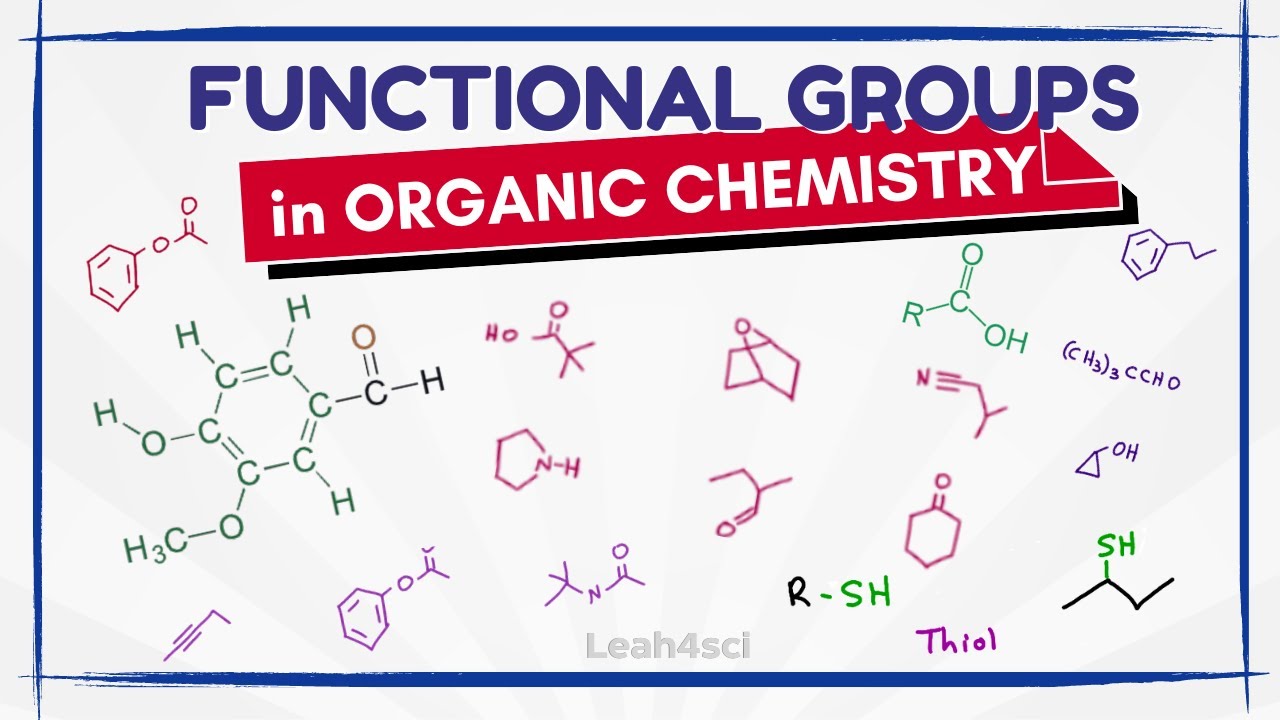
https://leah4sci.com/functional presents: Functional Groups with Memorization tips for organic chemistry. Alkane alkene alkyne, alkyl halide, alcohol, ether, epoxide, ketone, aldehyde, carboxylic acid and more
This video breaks down the common functional groups in organic chemistry, from the 'R' group to carbon chains, amines, alkyl halides, alcohols, thiols, ketones, aldehydes, carboxylic acids, esters, amides, phenyl vs phenol and more. Learn what the functional groups look like, how to identify them, and tricks to help you memorize groups for organic chemistry.
Struggling with Organic chemistry? Grab my free ebook '10 Secrets To Acing Organic Chemistry' http://leah4sci.com/orgo-ebook/
Resources mentioned in this video:
- Functional Groups Cheat Sheet https://leah4sci.com/organic-c....hemistry-functional-
- Functional Groups Practice Quiz https://leah4sci.com/organic-c....hemistry-functional-
- Pencil Trick to identify primary/secondary/tertiary/quaternary groups https://leah4sci.com/pencil-tr....ick-for-classifying-
- Naming Organic Compounds video series: https://leah4sci.com/naming-or....ganic-compounds-iupa
I offer private online tutoring for organic chemistry and MCAT prep. Details on my website: https://leah4sci.com/organic-chemistry-tutor/
for questions and comments, find me on social media here:
Facebook: https://www.facebook.com/Leah4sci
Twitter: https://twitter.com/Leah4Sci
Instagram: Instagram.com/leah4sci
Pinterest: https://www.pinterest.com/leah4sci/
The organic molecules that make up life on Earth are more than just the 2-D structures we’ve been drawing so far. Molecules have 3-D shapes that help us understand what they can do. In this episode of Crash Course Organic Chemistry, we’ll learn how orbital hybridization and valence bond theory can help us explain 3D molecular structures and about constitutional and geometric isomers.
Episode Sources:
Discovery of DNA Double Helix: Watson and Crick | Learn Science at Scitable
Citation: Pray, L. (2008) Discovery of DNA structure and function: Watson and Crick. Nature Education 1(1):100
https://www.nature.com/scitabl....e/topicpage/discover
Nature 1953.
Watson J.D., Crick F.H. A structure for deoxyribose nucleic acid. Nature 1953;171:737–738.
Harding, S. E., Channell, G., & Phillips-Jones, M. K. (2018). The discovery of hydrogen bonds in DNA and a re-evaluation of the 1948 Creeth two-chain model for its structure. Biochemical Society transactions, 46(5), 1171–1182. doi:10.1042/BST20180158
Series Sources:
Brown, W. H., Iverson, B. L., Ansyln, E. V., Foote, C., Organic Chemistry; 8th ed.; Cengage Learning, Boston, 2018.
Bruice, P. Y., Organic Chemistry, 7th ed.; Pearson Education, Inc., United States, 2014.
Clayden, J., Greeves, N., Warren., S., Organic Chemistry, 2nd ed.; Oxford University Press, New York, 2012.
Jones Jr., M.; Fleming, S. A., Organic Chemistry, 5th ed.; W. W. Norton & Company, New York, 2014.
Klein., D., Organic Chemistry; 1st ed.; John Wiley & Sons, United States, 2012.
Louden M., Organic Chemistry; 5th ed.; Roberts and Company Publishers, Colorado, 2009.
McMurry, J., Organic Chemistry, 9th ed.; Cengage Learning, Boston, 2016.
Smith, J. G., Organic chemistry; 6th ed.; McGraw-Hill Education, New York, 2020.
Wade., L. G., Organic Chemistry; 8th ed.; Pearson Education, Inc., United States, 2013.
***
Watch our videos and review your learning with the Crash Course App!
Download here for Apple Devices: https://apple.co/3d4eyZo
Download here for Android Devices: https://bit.ly/2SrDulJ
Crash Course is on Patreon! You can support us directly by signing up at http://www.patreon.com/crashcourse
Thanks to the following patrons for their generous monthly contributions that help keep Crash Course free for everyone forever:
Eric Prestemon, Sam Buck, Mark Brouwer, Zhu Junrong, William McGraw, Siobhan Sabino, Jason Saslow, Jennifer Killen, Matija Hrzenjak, Jon& Jennifer Smith, David Noe, Jonathan Zbikowski, Shawn Arnold, Trevin Beattie, Matthew Curls, Rachel Bright, Khaled El Shalakany, Ian Dundore, Kenneth F Penttinen, Eric Koslow, Timothy J Kwist, Indika Siriwardena, Caleb Weeks, Haixiang Liu, Nathan Taylor, Andrei Krishkevich, Sam Ferguson, Brian Thomas Gossett, SR Foxley, Tom Trval, Justin Zingsheim, Brandon Westmoreland, dorsey, Jessica Wode, Nathan Catchings, Yasenia Cruz, christopher crowell
--
Want to find Crash Course elsewhere on the internet?
Facebook - http://www.facebook.com/YouTubeCrashCourse
Twitter - http://www.twitter.com/TheCrashCourse
Tumblr - http://thecrashcourse.tumblr.com
Support Crash Course on Patreon: http://patreon.com/crashcourse
CC Kids: http://www.youtube.com/crashcoursekids
All the common functional groups of organic chemistry are explained with the generic structural formula and an example. At the end, I also go over a common mistake students make and how to avoid getting it wrong on your next ochem test.
📘 FREE ORGANIC CHEMISTRY SURVIVAL GUIDE
https://melissa.help/freeochemguide
💯 HERE'S HOW TO PASS ORGANIC CHEMISTRY 🎉
https://chemmunity.info/youtube
👉 MORE CHEMISTRY RESOURCES I CREATED 👈
https://melissamaribel.com/
🎓 CHECKOUT MY COMPLETE CHEMISTRY GUIDES:
📕 Thermochemistry Guide
https://melissa.help/thermonotes
📗 Acids and Bases Guide
https://melissa.help/acidbase1notes
📘 Naming Compounds and Acids Guide
https://melissa.help/namingnotes
📙 Dimensional Analysis, Significant Figures, and Density Guide
https://melissa.help/sigfignotes
📕 Gas Laws Guide
https://melissa.help/gaslawsnotes
📗 Stoichiometry Guide
https://melissa.help/stoichnotes
📘 Redox Reactions Guide
https://melissa.help/redoxnotes
📙 Molarity Guide
https://melissa.help/molaritynotes
📕 Limiting Reactants Guide
https://melissa.help/limreactnotes
📗 Lewis Structures Guide
https://melissa.help/lewisnotes
📘 Kinetics Guide
https://melissa.help/kineticsnotes
📙 Titrations Guide
https://melissa.help/titrations
📕 Matter, Atomic Structure, Empirical and Molecular Formulas Guide
https://melissa.help/matterguide
🙌 This was my go-to homework help when I was in school. Chegg Study is one of my favorites.
https://melissa.help/cheggstudy
📚 I made the mistake of buying all of my textbooks, I wish I had the option of renting them. Thankfully you do, with Chegg Textbook Rentals.
https://melissa.help/cheggbooks
💁♀️ HI I'M MELISSA MARIBEL
I help students pass Chemistry and Organic Chemistry. I used to struggle with this subject, so when I finally graduated with a bachelor's degree in Chemistry, I became a tutor so that you wouldn't have to struggle like I did. I know that with the right help, YOU CAN LEARN ANYTHING!
DISCLAIMER: Some links in the description are affiliate links, which means that if you buy from those links, I’ll receive a small commission. This helps support the channel and allows me to continue making videos like this. Thanks for the support!
Hey everyone! This is a short video explaining the whole of Organic Chemistry for AS Level in 40 minutes. Hope you like it! This is part 1 of the 2 part series.
Support me on Patreon and unlock bonus perks!
https://www.patreon.com/CAIEPapersSolved
00:00 Intro
00:41 Part 1: Introduction to General Terms in Organic Chemistry + Isomerism
10:33 Part 2: Reactions and Production of Functional Groups Including Reagents and Conditions
35:10 Part 3: All Reaction Mechanism Involved in AS ORGANIC
For more content, like the video and subscribe to the channel! Please let me know which papers you want me to solve next.
Follow me:
Website: https://caiepaperssolved.com/
Twitter: https://twitter.com/CAIEPaperSolved
YouTube: https://www.youtube.com/c/CAIEPapersSolved
Alkanes are comprised of a series of compounds that contain carbon and hydrogen atoms with single covalent bonds. This group of compounds consists of carbon and hydrogen atoms with single covalent bonds. Also, comprises a homologous series having a molecular formula of C nH2n+2.
Homologous series has enabled scientists and engineers to study different organic compounds systematically. They can predict the properties of organic compounds belonging to a particular homologous series based on the data available from the other members of the same series. The study of organic compounds has been simplified.
Do like, share, subscribe, learn and spread knowledge. 😊
#chemistry #Hydrocarbons #organicchemistry #alkanes #homologous #series #chemistry #organicchemistry #generalorganicchemistry #goc #class11chemistry #class12chemistry #chemistrykustad #Amazingchemistryvideo, #Amazingchemistryvideos, #Amazing, #Chemistry, #Video, #Amazingchemistryvideo, #Amazingchemistryvideos, #Jeechemistry, #Jee, #Chemistry, #Jeechemistryunacademy, #Jeechemistrylecture, #Jeechemistrythermodynamics, #Jeechemistryclass11, #Jeechemistryoneshot, #Jeechemistryclass11chapter1, #Jeechemistrycrashcourse, #Jeechemistryimportanttopics, #Jeechemistrystrategy, #Jeechemistrysuryabhansir, #Jeechemistrybooks, #Jeechemistrytricks, #Jeechemistry21, #Neetchemistry, #Neet, #Chemistry, #Neetchemistrylectures, #Neetchemistrymcqs, #Neetchemistrytricks, #Neetchemistrybewise, #Neetchemistrylecturesclass11chapter1, #Neetchemistrylecturesclass12, #Neetchemistryoneshot, #Neetchemistryalllectures, #Neetchemistrysyllabusorganicinorganicphysical, #Neetchemistrylecturesmoleconceptunacademy, #Neetchemistrycrashcourse22neetchemistrysyllabus22neetchemistrybyte
Naming Alkenes, IUPAC Nomenclature Practice, Substituent, E Z System, Cycloalkenes Organic Chemistry
This organic chemistry video explains how to name alkenes using the iupac nomenclature system. It contains examples with substituents, cycloalkenes, cis trans isomers and naming alkenes using the e z system.
Stereochemistry R/S Configuration:
https://www.youtube.com/watch?v=yzfcrwJ37kI
Optical Activity & Specific Rotation:
https://www.youtube.com/watch?v=duGxp_XZzvw
SN1, SN2, E1, E2 Reaction Mechanisms:
https://www.youtube.com/watch?v=hz-fSXifP9w
SN1, SN2, E1, E2 - Practice Test:
https://www.youtube.com/watch?v=qTc2uud7TVU
Alkene Reactions Review:
https://www.youtube.com/watch?v=lKROX1C0JRs
________________________________
Alkyne Reactions Review:
https://www.youtube.com/watch?v=p6TLF92lyuI
Organic Chemistry PDF Worksheets:
https://www.video-tutor.net/orgo-chem.html
Organic Chemistry 1 Exam 2 Playlist:
https://bit.ly/3PKEApB
Organic Chemistry 1 Final Exam Review:
https://www.youtube.com/watch?v=ej2pSWw6U3w
Full-Length Videos and Worksheets:
https://www.patreon.com/MathSc....ienceTutor/collectio